Internal QMS Corporate Function Audits:
(Pharmaceutical, Medical Device, Biotech, and Healthcare Suppliers)
Examples of Internal QMS Corporate Functions Audits we conduct:
 Deviation and CAPA Management Deviation and CAPA Management
|
 Computer System Validation Computer System Validation
|
 Documents and Change Control Documents and Change Control
|
 Supplier Management Supplier Management
|
 Complaint Management Complaint Management
|
 Inspection and Testing Inspection and Testing
|
 Professional Development & Training Professional Development & Training
|
 Facilities and Equipment Facilities and Equipment
|
 Medical Device Servicing Medical Device Servicing
|
 Technology Transfer Technology Transfer
|
 Design Controls (Device) Design Controls (Device)
|
 Safety & Pharmacovigilance Safety & Pharmacovigilance
|
 Device Master Records (Device) Device Master Records (Device)
|
 Risk Management Risk Management
|
 Device History Records (Device) Device History Records (Device)
|
 Auditing & Self-Assessment Auditing & Self-Assessment
|
 Management Reviews Management Reviews
|
 Recalls & Field Action Mgmt. Recalls & Field Action Mgmt.
|
 Warehousing / Storage Warehousing / Storage
|
 Packaging & Label Controls Packaging & Label Controls
|
 Stability Program (Pharma) Stability Program (Pharma)
|
 Shipping and Distribution Shipping and Distribution
|
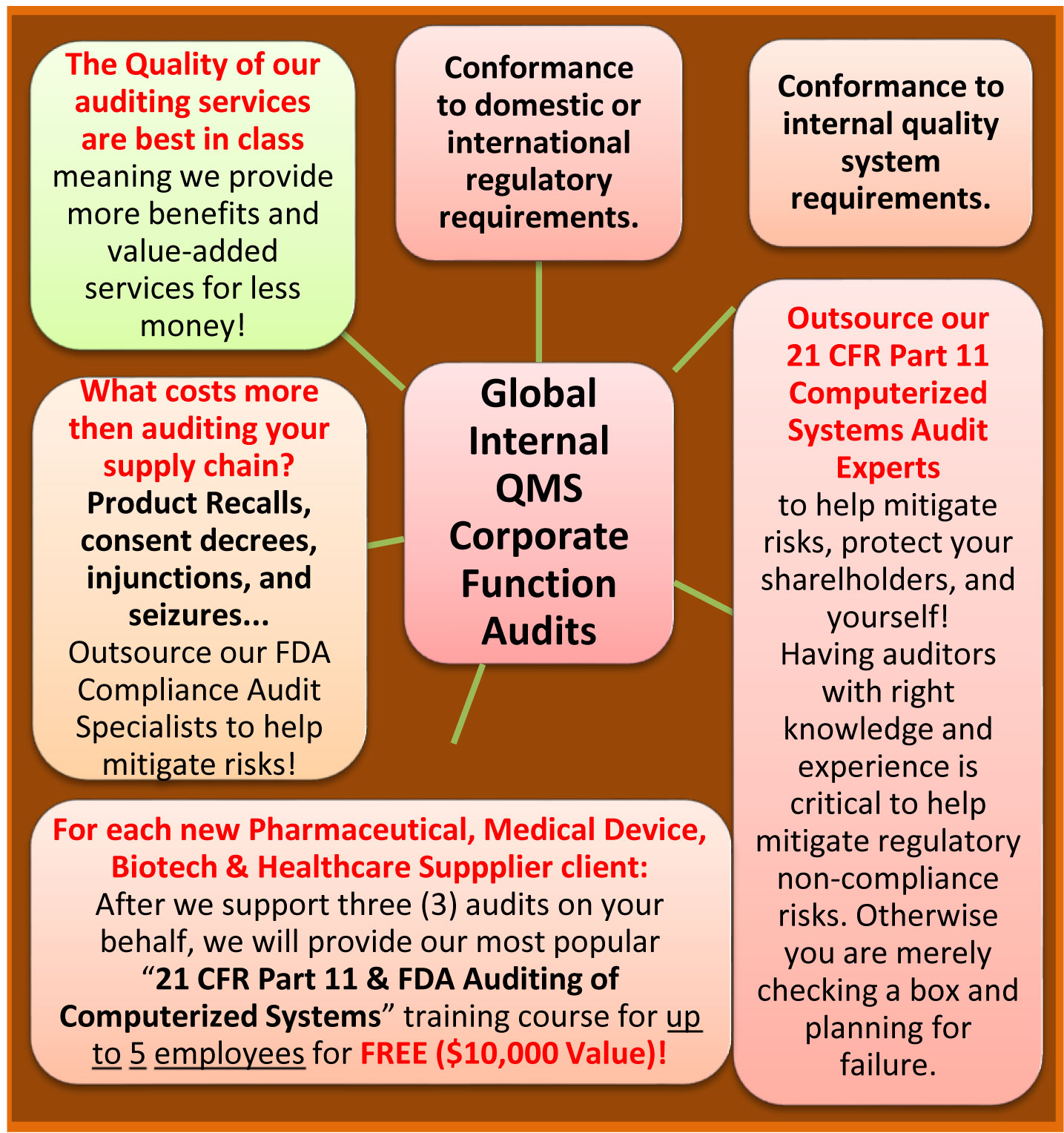
The Quality of our auditing services are best in class meaning we provide more benefits and value-added services for less money!
We provide high-caliber, FDA Compliance Specialists with 15+ years of regulated industry auditing experience; we provide a cost-effective, fixed price audit for each audit we support; our fixed price audit includes: pre-audit preparation; audit planning; travel time (U.S. only); the audit itself; daily audit debriefs; the final audit report with one review cycle; and we assign a dedicated U.S. based audit administrator for every audit we support. Outsource our highly experienced auditors today!
FREE FDA Compliance Training $10,000 Value!
For each new Pharmaceutical, Medical Device, Biotechnology, and Healthcare Supplier client - After we support three (3) audits on your behalf, we will provide our most popular "21 CFR Part 11 & FDA Auditing of Computerized Systems" online training class for up to 5 employees for FREE ($10,000 value)!