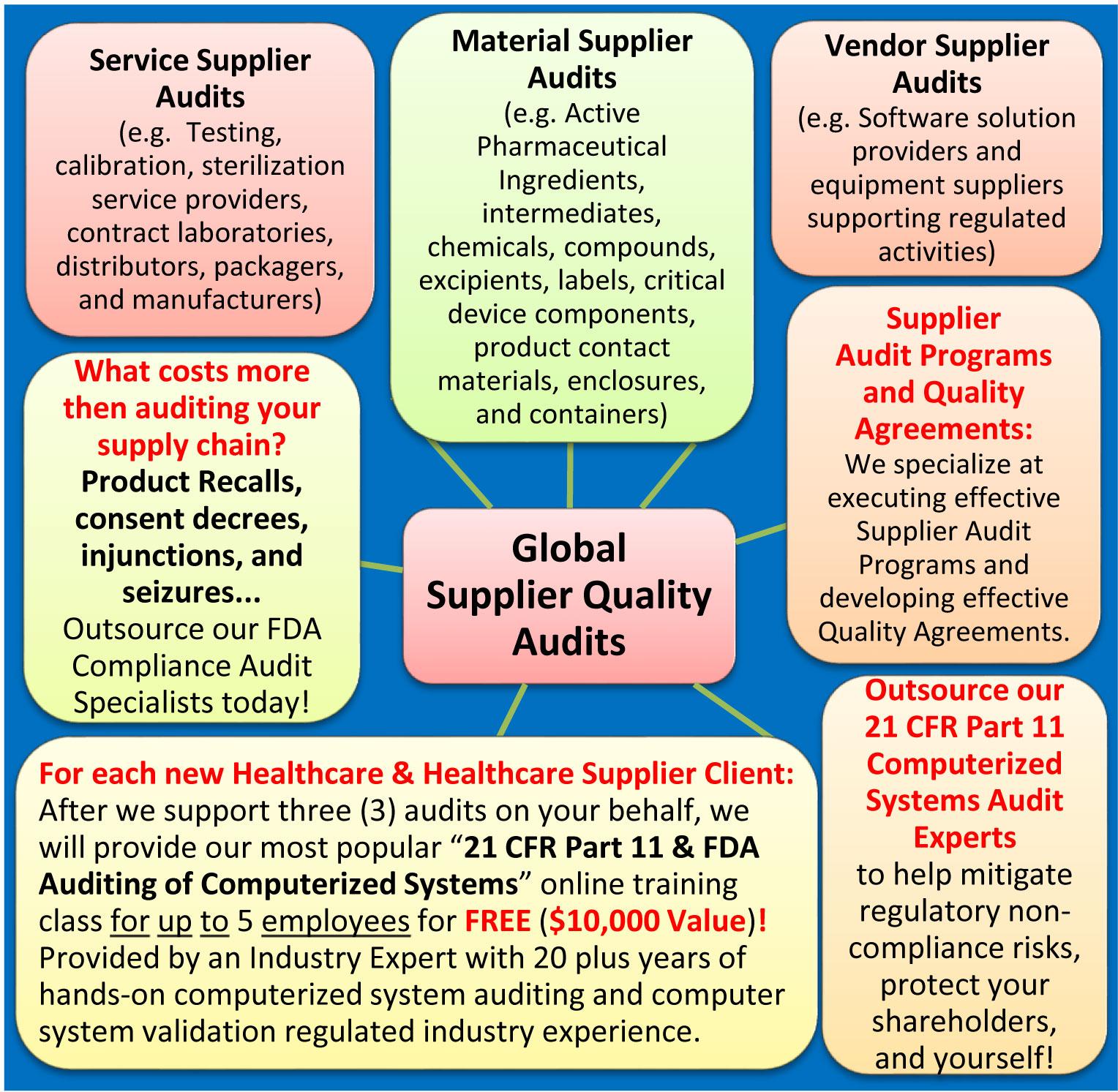
Supplier Quality Audits:
(Pharmaceutical, Medical Device, and Biotechnology Industries)
Supply chain globalization is a continuous challenge and many regulated companies must manage an increasingly challenging and demanding internal and/or supplier audit program without adequate resources. Outsource our highly experienced auditors today!
Fully qualified auditors experienced with regulations and standards:
(e.g. ISO 9001, ISO 13485, ISO 14971, 21 CFR Part 210, 21 CFR Part 211, 21 CFR Part 11, EU Directive 2003/94/EC, Health Canada Food and Drug Regulations, 21 CFR Part 820, 21 CFR Part 4, EU MDD 93/42/EEC [amended 2007/47/EC], EU Annex 11, SOR/98/282, Japan’s PMD Act, ICH Q6A, ICH Q9, ICH Q10, 21 CFR Part 50, 21 CFR Part 54, 21 CFR Part 56, ICH E6, ICH E2A, ICH E8, PIC/S PE009-11, and GAMP).
The Quality of our auditing services are best in class meaning we provide more benefits and value-added services for less money!
We provide high-caliber, FDA Compliance Specialists with 15+ years of regulated industry auditing experience; we provide a cost-effective, budget friendly, fixed price audit for each audit we support; our fixed price audit includes: pre-audit preparation; audit planning; travel time (U.S. only); the audit itself; daily audit debriefs; the final audit report with one review cycle; and to complement our professional auditing services, we assign a dedicated U.S. based audit administrator for every audit we support.
FREE FDA Compliance Training $10,000 Value!
For each new Pharmaceutical, Medical Device, Biotechnology, and Healthcare Supplier client - After we support three (3) audits on your behalf, we will provide our most popular “21 CFR Part 11 & FDA Auditing of Computerized Systems” online training class for up to 5 employees for FREE ($10,000 value)!