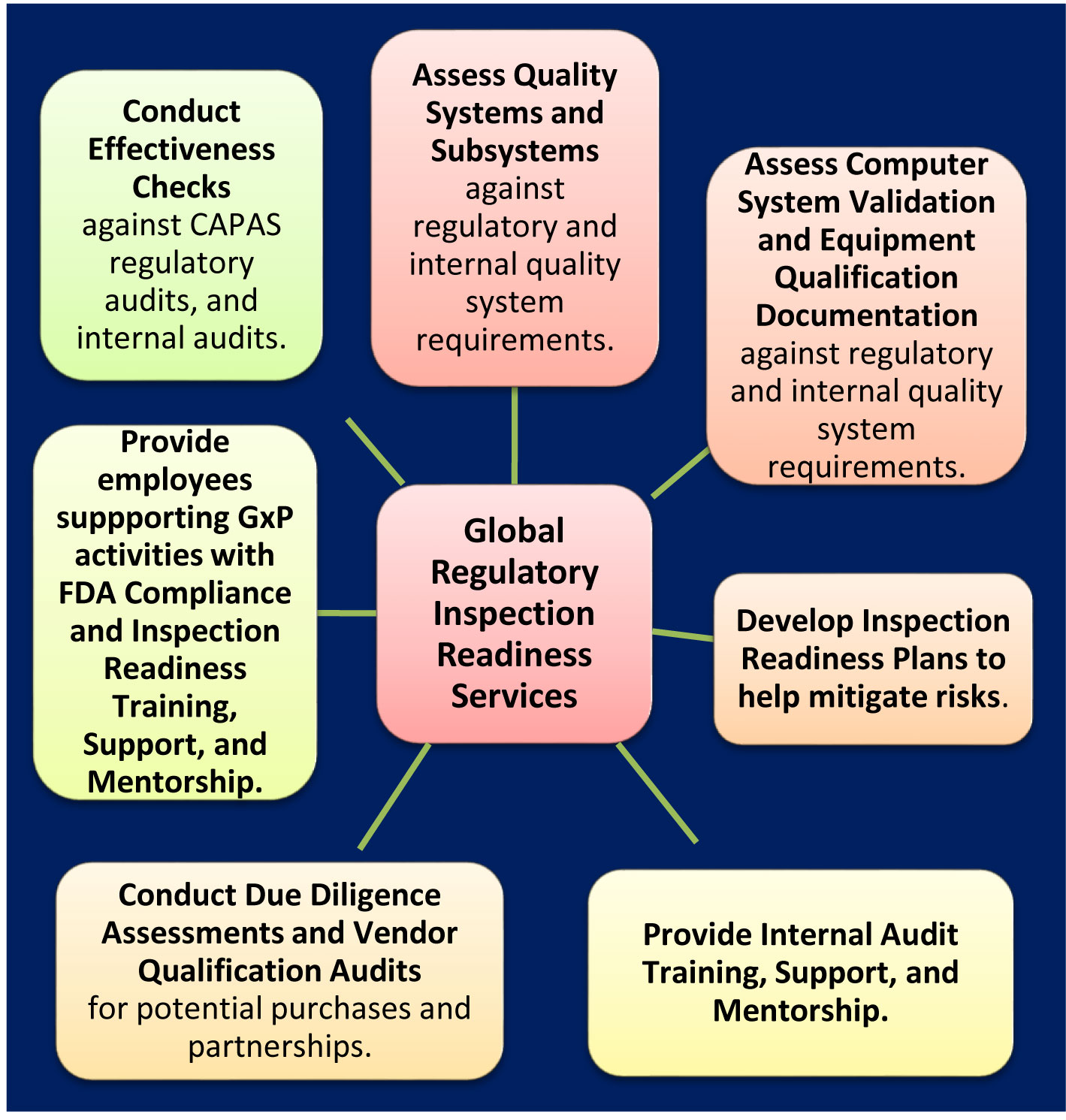
Regulatory Inspection Readiness Services:
(Pharmaceutical, Medical Device, Biotech, and Healthcare Suppliers)
What costs more than proactively investing in Inspection Readiness?
483 Remediation; product recalls, consent decrees, injunctions, seizures, and expensive delays in FDA approval (which also has a rippling effect - loss of jobs). Proactively plan and manage inspection readiness to mitigate risks. Outsource our highly experienced FDA Compliance Specalists today!
Benefits And Value Of Our Regulatory Inspection Readiness Services Include, But Are Not Limited To:
- FDA Compliance Specialist audits of internal QMS functional areas & suppliers strengthen internal auditing capabilities.
- Proactively identifying procedural, technical, personnel, or documentation gaps helps mitigate unnecessary / unwanted regulatory observations and facilitate FDA approval.
- Proactively training manufacturing quality personnel results in reduced wasted time, reduced materials, reduced regulatory non-compliance issues, improved quality and inspection readiness.
- FDA Compliance Specialist documentation review to mitigate risks (e.g. internal QMS (policies, SOPs); Effectiveness Checks of CAPA commitments; CSV documentation; compliance training).
- Develop Inspection Readiness Plans to help mitigate non-compliance issues (e.g. QMS policies, SOPs, and work instructions; computerized systems; computer system validation; processes; training; internal and supply chain auditing).